
Heat Revision
Introduction.
What is temperature?- Temperature is a measure of the average kinetic energy of the particles in an object.
[This implies an absolute zero to temperature, but no maximum.]
Units: - Kelvin (K), degrees centigrade (°C), degrees celsius (°C)
What is heat?
- Heat is a form of energy, and is subject to the conservation of energy principal.
- It is a measure of the total kinetic and potential energy contained by the molecules in an object.
- Heat is what is transferred between two objects when there is a temperature difference.
Units: - Joules (J)
Heat transfer:-
- Conduction,
- heat is transferred between particles by vibrations, without the particles changing position.
- Convection,
- heat is transferred by the movement of particles from one place to another.
- Radiation,
- heat is transferred by electromagnetic radiation.
Measuring temperature.
Name three different types of thermometer and briefly explain the principle on which they work.- Expansion of a material - bimetal, liquid in glass, change in gas pressure.
- Change in electrical resistance - thermistor, diode, platinum.
- Generation of an electric current - thermocouple.
- Pyrometry - measurement of the wavelength of the em radiation emitted.
Calibration Fixed points.
0°C - crushed melting ice at standard pressure.
100°C - steam from boiling water at standard pressure.
0.010°C - the triple point of water at standard pressure.
231.928°C - the freezing point of pure tin.
Heat Capacity.
This is the amount of energy needed to raise the temperature of an object by 1K.In practice, this is of little use for general measurements since the heat capacity depends on the mass of the object and the material it is made from.
A more useful measure is Specific Heat Capacity (c).
This is a measure of the energy (Q) needed to raise the temperature of 1kg of a material by 1K.
Units Joules per kilogram per kelvin - Jkg-1K-1
Q = m c Δθ
Δθ = change in temperature
Some common specific heat capacities, in Jkg-1K-1:-
Water 4180, Ice 2100, Copper 390, Iron 450, Glass 840, Wood 1700, Concrete 3300, Brick 840
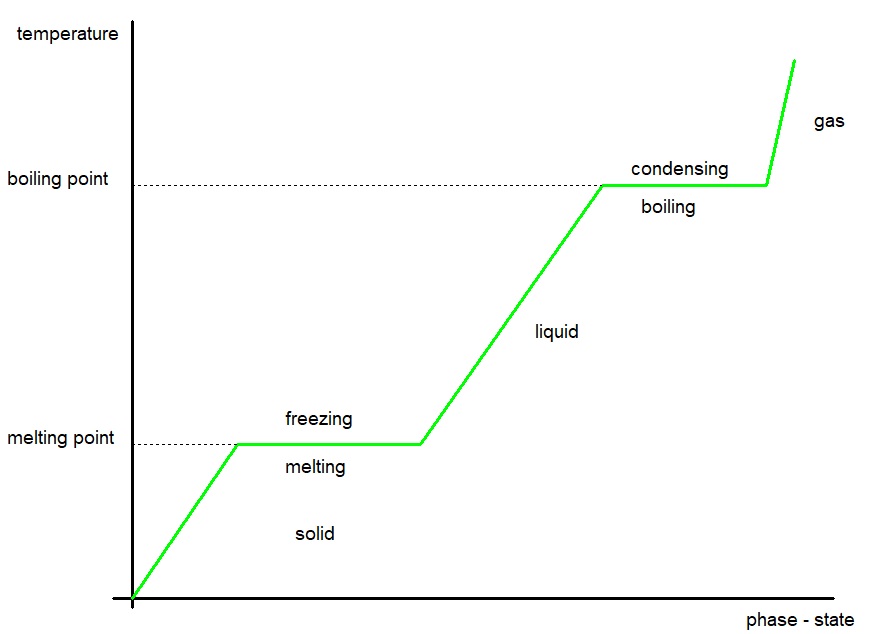
Phase Changes (Change of State).
There are four phases (states) of matter - solid, liquid, gas, plasma.A phase change is when matter changes from one state to another.
A phase change occurs when enough energy is supplied (or lost) from a system or the pressure changes.
During a phase change, the temperature of the matter remains constant.
Latent heat is the energy gained or lost during a phase change.
Specific latent heat (l) is the energy gained or lost during a phase change per kg or matter Jkg-1.
Q = m l
Specific latent heat of fusion - energy gained or lost with a phase change of solid/liquid.Specific latent heat of vaporisation - energy gained or lost with a phase change of liquid/gas.
Specific latent heat of vaporisation of water - 2.3×106Jkg-1
Experiments.
It is helpful to have experience of the following experiments.- Determination of the specific heat capacity of a solid, e.g. copper.
- Determination of the specific heat capacity of a liquid, e.g. water.
- Determination of the specific latent heat of fusion of a solid, e.g. ice, wax.
- Determination of the specific latent heat of vaporisation of a liquid, e.g. water.
For each experiment you need to be able to:-
- Describe/draw the equipment and how it is set up; (large, labelled diagram),
- Identify health and safety risks and how they are minimised/eliminated; (high and low temperatures, burns and scalds, electricity),
- What measurements you would make and how they would be recorded, including uncertainties; (tables, headings, number of measurements),
- How the measurements are processed to obtain a final value, including uncertainties; (The Physics, clearly set out calculations, graphs and scales for axes, uncertainties),
- Improvements to the experiment; (better insulation).
Questions.
NOTE Calculations should be set out in a clear and logical manner:-- This will help you to find any errors more easily and
- examiners to follow what you have done, and so award marks appropriately.
1). A 2kW electric copper kettle has a mass of 0.5kg when empty and a mass of 1.5kg when full of water at a temperature of 5°C. Calculate:-
(a) the amount of energy (in joules) needed to heat the water to boiling point,
(b) the time taken,
(c) the cost, if 1kWh of electricity costs 30p.
(Specific heat capacity, in Jkg-1K-1 of water is 4180 and copper is 390)
2). To measure the temperature of a gas flame, a piece of copper of mass 50g is heated in the flame until it reaches a steady temperature.
The copper is then put into a glass beaker of mass 300g containing 500g of water at a temperature of 12°C
The water is gently stirred until a steady temperature is reached, this being measured as 19.5°C
(a) Calculate the temperature of the copper when it was in the gas flame,
(b) Explain why the gas flame is likely to be hotter than the piece of copper,
(Specific heat capacity, in Jkg-1K-1 of water is 4180, copper is 390 and glass is 840)
3). While cooking a curry sauce on an electric cooker, a chef adds too much water.
If 1kWh of electricity costs 30p, calculate the cost of evaporating 70ml of excess water.
(Specific latent heat of vaporisation of water - 2.3×106Jkg-1)
4). Estimate the mass of ice from a freezer at -18°C is needed to cool 200ml of orange squash in a polystyrene cup from 20°C to 10°C.
(Specific latent heat of fusion of ice - 3.3×105Jkg-1)
(Specific heat capacity, in Jkg-1K-1 of ice is 2100 and orange squash is 4200)
5). Explain why a steam burn is much more dangerous than a burn from water at 100°C.
6). To determine the specific heat capacity of water, 200g of water is put into a copper beaker of mass 150g.
The copper beaker is insulated with a thick layer of polystyrene foam.
The arrangement is heated by a small electrical heater of negligable mass.
The temperature of the water is measured using a thermocouple thermometer, also of negligable mass.
The electrical heater uses a 12V supply and has a resistance of 4Ω.
Calculate:-
(a) the electrical energy supplied per minute to the water by the heater
(b) the rise in temperature of the water per minute.
(c) the time taken for the water to boil, if it started at a temperature of 10°C.
(Specific heat capacity, in Jkg-1K-1 of water is 4180 and copper is 390)
7). To estimate the specific latent heat of vaporisation of water an electric kettle of water is left boiling.
The mass of the kettle when it started to boil was 1.9kg and after 5 minutes it was 1.69kg.
The mains voltage was 235Vrms and the current passing through the kettle was 8.2Arms.
(a) Calculate a value for the specific latent heat of vaporisation of water.
(b) Suggest a reason for the difference between this value and the accepted value.
8). To determine the latent heat of fusion of ice, crushed ice from a freezer is added to a mass of water at a known temperature.
The ice is stirred with the water until a steady temperature is reached, which is then recorded.
The following measurements were taken:-
Mass of crushed ice added = 48g ± 0.5g
Mass of empty insulated glass beaker = 120g ± 0.5g
Mass of insulated glass beaker with water = 315g ± 0.5g
Temperature of crushed ice = -16°C ± 0.2°C
Initial temperature of glass beaker and water = 32°C ± 0.2°C
Final temperature of glass beaker and water = 10.6°C ± 0.2°C
Calculate the latent heat of fusion of ice and give estimate the likely uncertainty in your answer.